A collaborative study by Milan Němý from CIIRC CTU, Daniel Ferreira from Swedish Karolinska Institute (KI) and Stefan Teipel from the German Center for Neurodegenerative Diseases (DZNE) et al. introduced a new imaging marker of brain connectivity that appears to be a very early indicator of pathological changes in case of Alzheimer’s disease. This imaging marker could be capable of detecting alterations when formal neuropsychological tests and even standard clinical image-based markers fail. The latest study was published in the scientific journal Brain.
Alzheimer’s disease can be regarded as a continuum. Its very early stage usually includes people with positive signs that are characteristic for this disease, but without obvious symptoms. Among them there are also individuals with self-reported memory complants, who have a significantly increased risk of progressing to more serious stages – from mild cognitive impairment to fully developed dementia. Accurate and timely diagnosis of this group of patients is very important for the deployment of available treatment, which can delay the onset of further stages of the disease.
Listen to the report from the Czech Radio
The research focuses on neural cells in a strategic brain region – the cholinergic basal forebrain – and their long and slender projections (axons), which are selectively vulnerable to Alzheimer’s disease pathology. These nerve cells with long axons tend to die in a specific way, where the degeneration of the long nerve fibers forego the death of the cell body and the death of the entire cell. It is therefore useful to target these brain fibers for research into the very early stages of the disease. The importance of cholinergic neurons is also underlined by the fact that the only currently available treatment for Alzheimer’s disease in the Czech Republic is aimed at these cells.
Research on this topic includes three studies led by Prof. Olga Štěpánková (CIIRC CTU), Dr. Daniel Ferreira (KI) and Prof. Stefan Teipel (DZNE) in close cooperation with Milan Němý (CIIRC CTU). In their studies, the researchers examined brain fibers using a technique based on magnetic resonance imaging and its diffusion-weighted sequence.

From left: Daniel Ferreira (KI), Milan Nemy (CIIRC CTU), Stefan Teipel (DZNE) (KI website)
„This is a sequence sensitive to the microscopic water movement and able to capture the direction of brain fibers. Tracking the desired trajectories using this technique depends on a precise determination of the location of the cell bodies from which brain pathways originate and other important landmarks facilitating the tracking process. In this regard, the key partners were from the German Center for Neurodegenerative Diseases (DZNE), who supplied a high-resolution segmentation mask of the cholinergic basal nuclei of the forebrain, which was crucial to serve as a starting point for this imaging method„, explains Milan Němý, researcher from CIIRC CTU.
And he continues: “The aim of the first study was to localize these cholinergic pathways in healthy individuals. Specifically, we used data from a community-based study from Spain led by Dr. Daniel Ferreira (GENIC cohort). We observed that the pathways found using this non-invasive method actually replicated the location of brain pathways known so far only from post-mortem studies, and for the first time we visualised these pathways in a healthy, living brain.“ These results were successfully replicated and extended in a second study including an independent cohort – the Gothenburg H70 Birth Cohort Studies led by Professor Ingmar Skoog from the Gothenburg University.
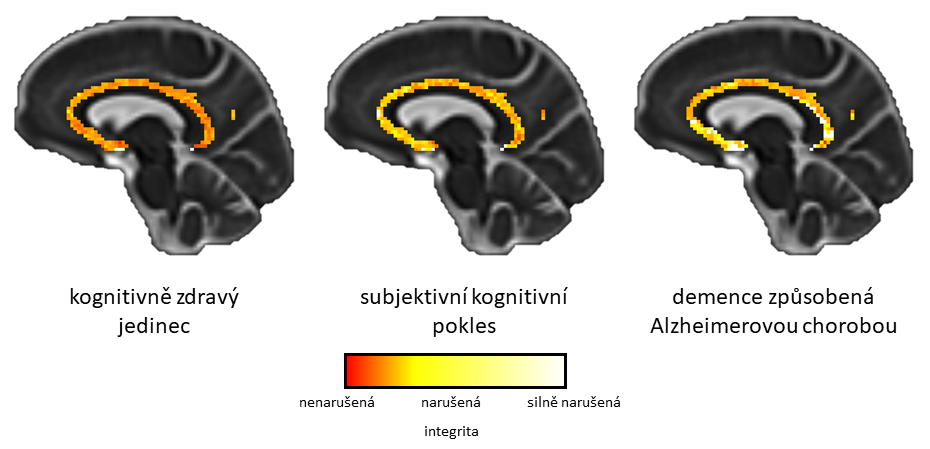
The spatial distribution of the integrity score of investigated cholinergic pathways shows significant changes already in the phase of subjective memory problems (from cognitively healthy individual on the left to individual suffering from the Alzheimer’s disease on the right)
Having identified the pathways and their location in the brain, the researchers investigated their microstructural properties in several diagnostic groups in the third and final study. This time, they had quite an extensive cohort at their hands with 172 patients with subjective cognitive decline, 66 patients with mild cognitive impairment, and 52 patients with Alzheimer’s disease dementia from the DZNE in Germany (DELCODE cohort). It was expected that significant differences would be found at least between patients with Alzheimer’s disease, i.e. with the most advanced stage of the continuum, and congnitively healthy individuals.
“We first focused on the overall integrity of the cholinergic pathways. Not only did we observe significant differences between patients with Alzheimer’s disease dementia and healthy individuals, but contrary to our assumption, we found that in individuals who subjectively perceive their memory problems, but at the same time these problems were not detected by standard psychological tests or other used methods, there were already significant changes!”, Milan Němý describes the surprising findings. Moreover, individuals with subjective cognitive decline did not differ from healthy subjects in cerebrospinal fluid-based biomarkers, a set of biomarkers currently used in specialized clinics in the Czech Republic to support the clinical diagnosis of Alzheimer’s disease. These results were demonstrated both in the whole clinical cohort but also in the amyloid-stratified subsample, suggesting that the integrity of cholinergic pathways, as captured by this method, might be an indicator of very early changes in the Alzheimer’s disease continuum.
„In this cross-sectional study, we were able to uncover the spatial distribution of the differences in the white matter integrity of the cholinergic pathways across the individual stages of the disease. Already in the preclinical stage of Alzheimer’s disease, this posterior cholinergic white matter of the brain showed early regional vulnerability. With the more advanced stages of the disease, these differences were even more pronounced. In this way, we made the first step for the development of a promising non-invasive biomarker of the earliest stages of Alzheimer’s disease„, Milan Němý summarizes the achieved results.
This latest study was published online in October 26, 2022 in the scientific jurnal Brain.